Advanced Trial Management for Sponsors and CROs
Maximize efficiencies, improve trial management, and reduce costs with DecenTRIAL.
DIRECT DATA CAPTURE
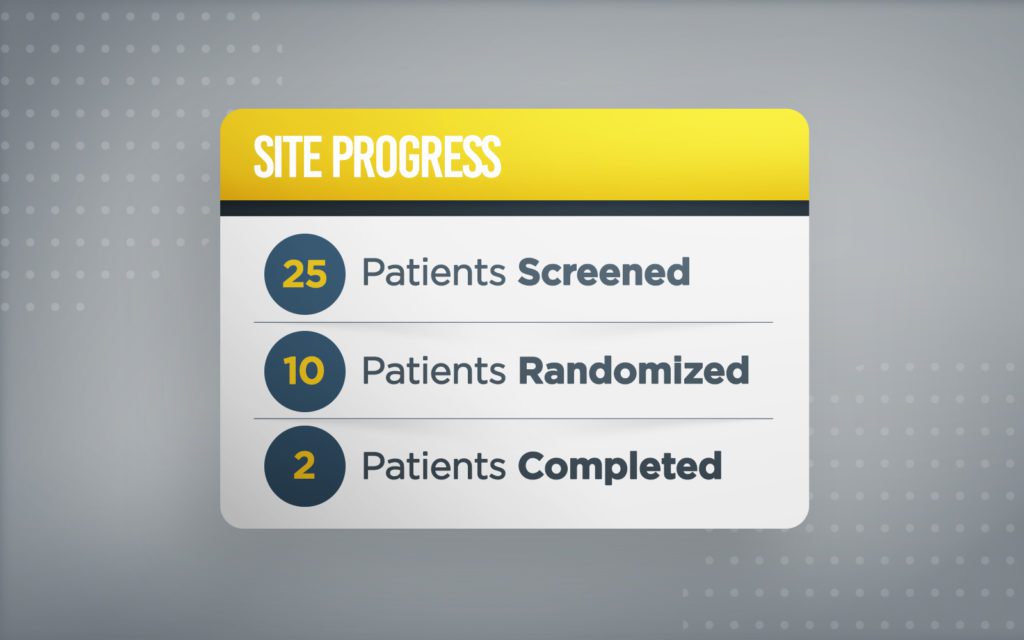
Free yourself from traditional EDC systems. Manage clinical trials from start to finish on a single platform.
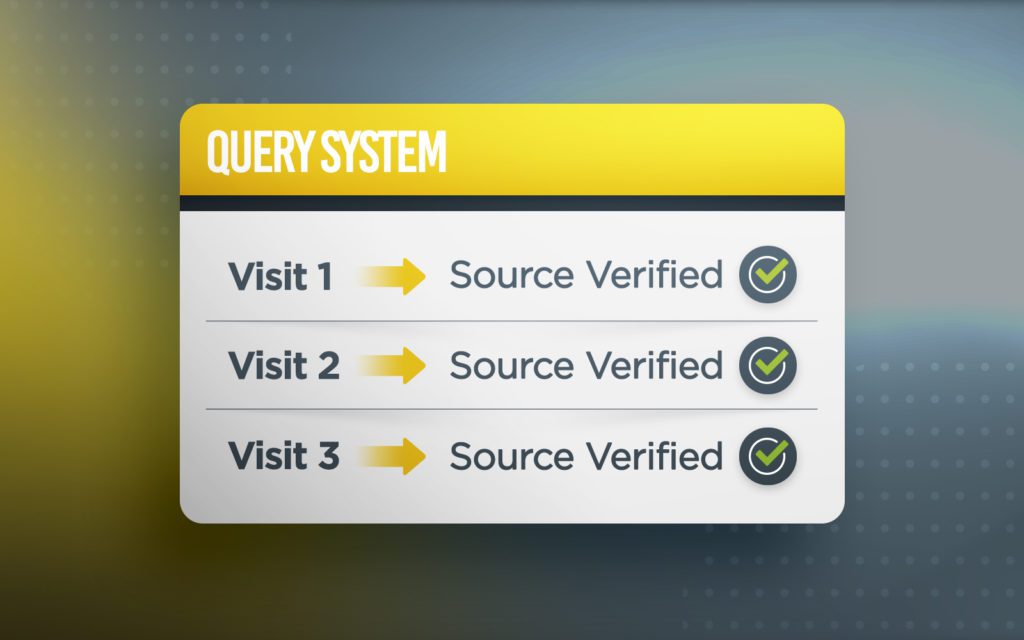
Powerful Remote Monitoring From Anywhere!
Manage clinical trials from start to finish on a single platform.


FLEXIBLE SOLUTIONS
Customize a solution that fits your specific needs. The only limit is your imagination!
REQUEST A FREE DecenTrial DEMO
[nf-popup id=15452]