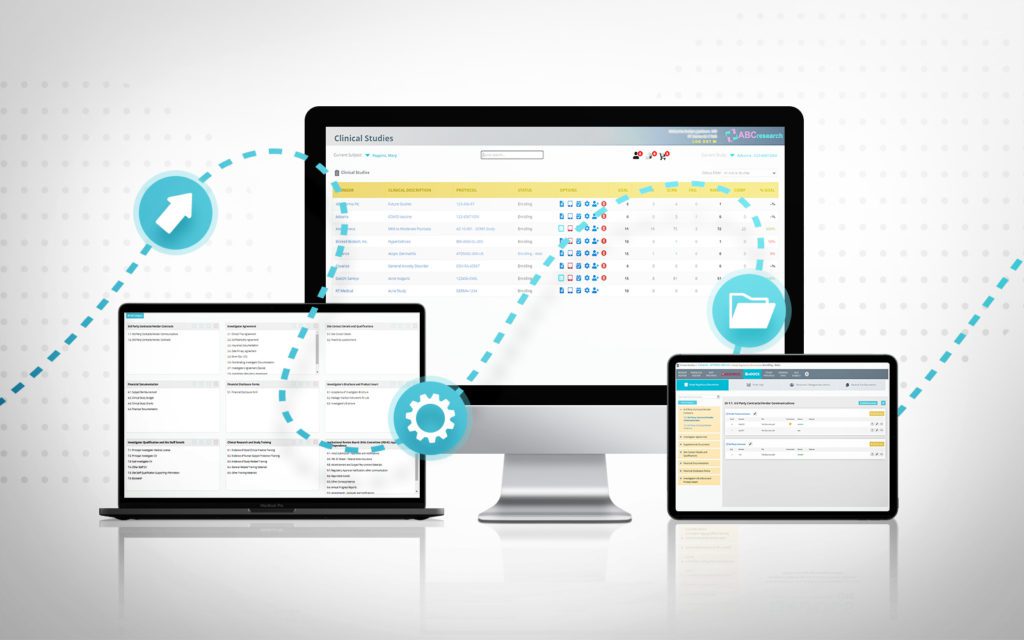
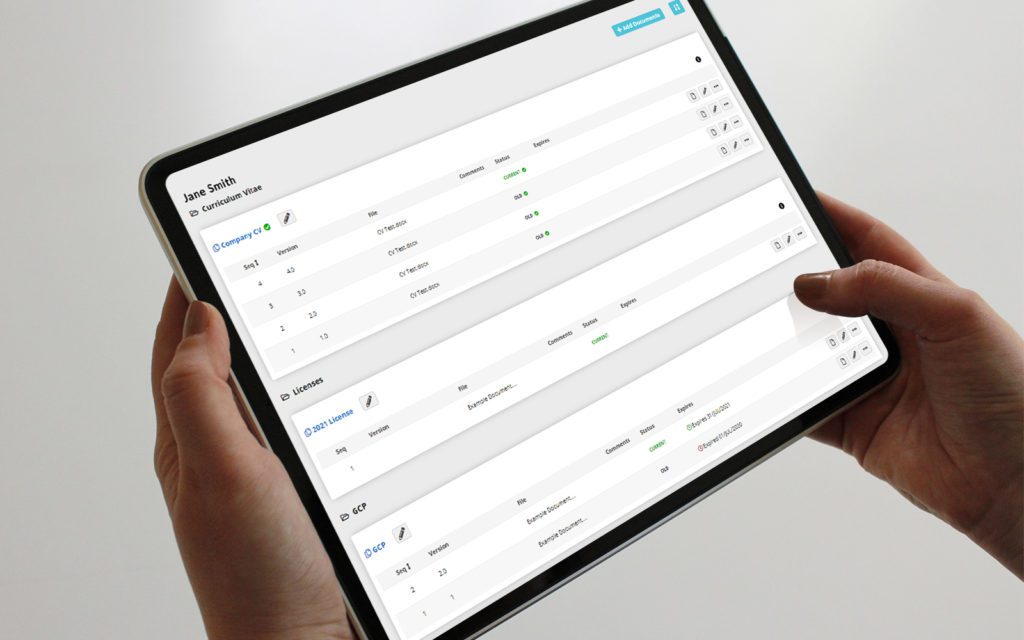
eRegulatory Binders for clinical trials
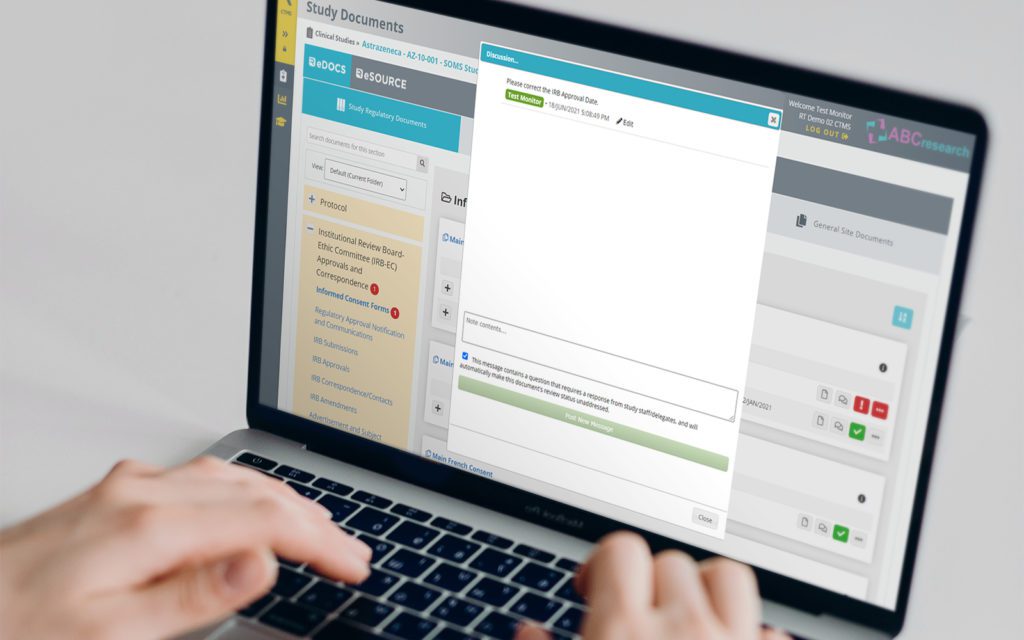
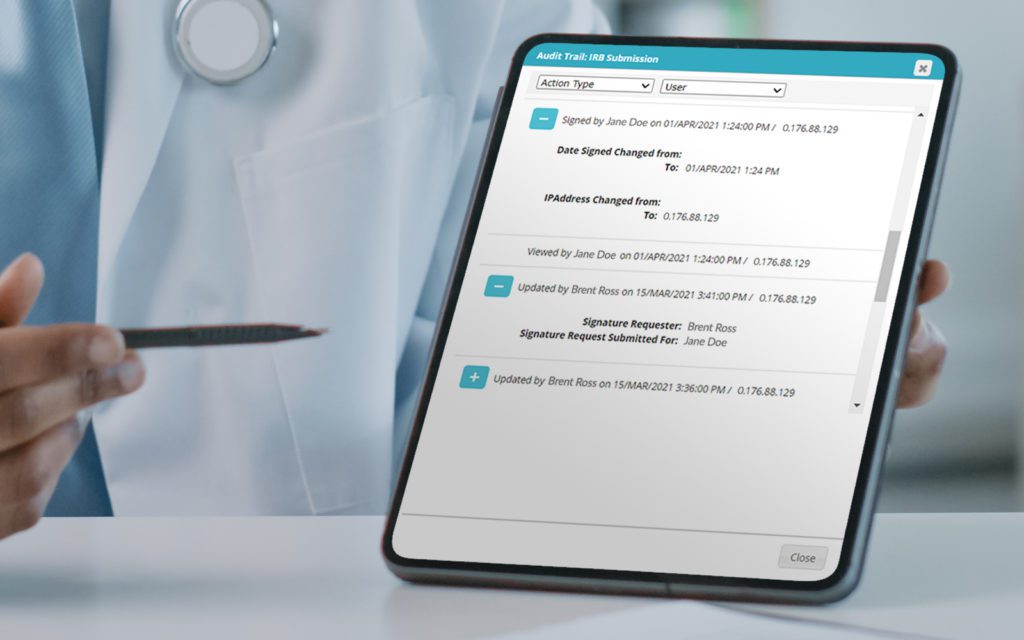
RealTime's eReg solution offers one secure location for quick and easy access to investigator site files (eISF) for staff, doctors, monitors and auditors.

AVAILABLE ONLY IN eDOCS!
Platform Integration
RealTime's eRegulatory solution fits seamlessly within the first and only SOMS on the market.
Download the Outlook Add-In
RealTime’s proprietary Add-In provides the ability to drag and drop documents and email directly into your eRegulatory binders.
Current Version: 1.2.0053

FLEXIBLE SOLUTIONS
Customize a solution that fits your specific needs. The only limit is your imagination!
REQUEST A FREE eDOCS DEMO
[nf-popup id=15440]