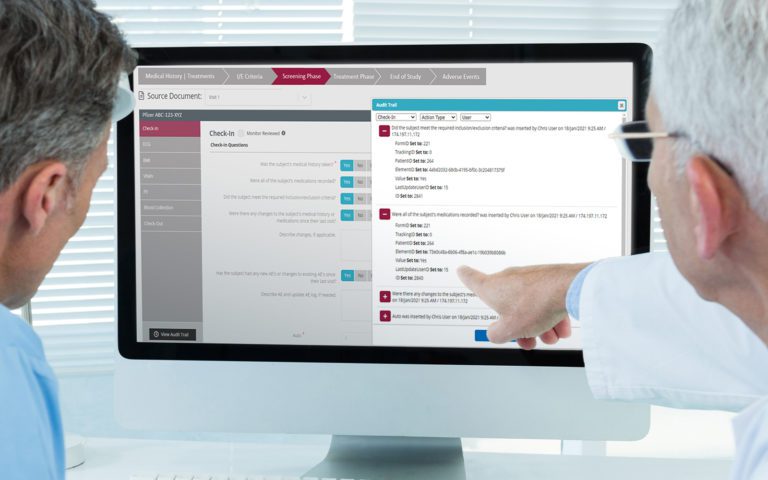
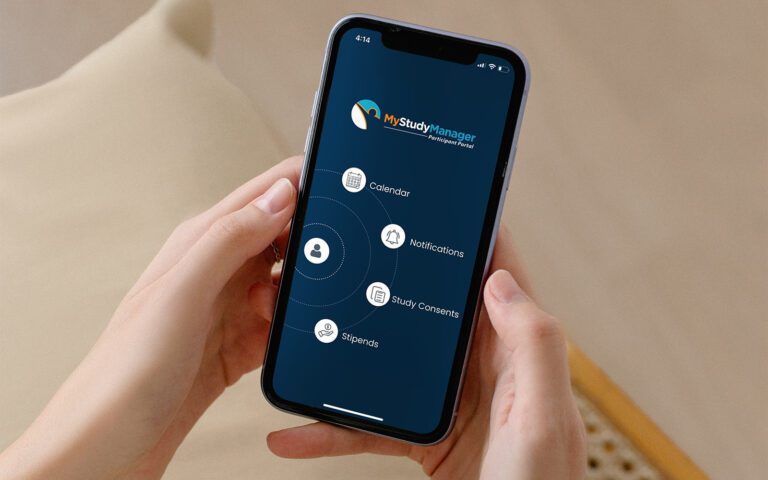
Platform Integration
RealTime-eSOURCE fits seamlessly within the first and only SOMS on the market.
FLEXIBLE SOLUTIONS
Customize a solution that fits your specific needs. The only limit is your imagination!
REQUEST A FREE eSOURCE DEMO
[nf-popup id=15431]